co2 bond angle and shape|CO2 Lewis Structure, : iloilo A quick explanation of the molecular geometry of CO2 including a description of the CO2 bond angles. We can see that there are only two atoms attached to the central Carbon (C) atom and. JOHN O’SHEA and Anthony Barry are now considered to be the leading contenders to replace Stephen Kenny according to Ladbrokes. The 118-times capped legend as well as the highly-rated Bayern M.
PH0 · Molecular Geometry Of CO2: How Lewis Structure Predicts
PH1 · Lewis Structure of Carbon Dioxide
PH2 · Geometry of Molecules
PH3 · CO2 Molecular Geometry and Bond Angles (Carbon Dioxide)
PH4 · CO2 Molecular Geometry and Bond Angles
PH5 · CO2 Lewis Structure, Molecular Geometry, Molar Mass & Hybridization
PH6 · CO2 Lewis Structure, Molecular Geometry, Molar
PH7 · CO2 Lewis Structure, Molecular Geometry and Hybridization
PH8 · CO2 Lewis Structure, Hybridization, Molecular
PH9 · CO2 Lewis Structure,
PH10 · CO2 Lewis Structure Molecular Geometry, CO2 Lewis
PH11 · 10.2: VSEPR Theory
The influx of visitors caused the Philippines government to temporarily close Boracay to tourists for six months. This 'rehabilitation' period, was used to restore the island to its former glory and it has since reopened with a limit on the number of daily visitors.The centre of the action is dreamy White Beach, a 4km, postcard-perfect stretch .
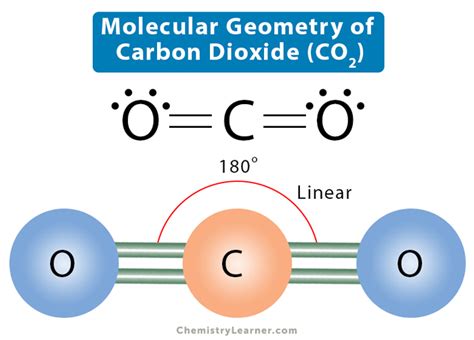
co2 bond angle and shape*******Hence CO2 has a linear molecular geometry with the bond angles of 180 degrees and symmetric distribution of electrons. Summary. To summarize this blog, we can say that Carbon Dioxide has a linear molecular geometry. It has an sp hybridization and has bond angles of 180 degrees. Tingnan ang higit paOne needs to know the Lewis structure in order to understand the molecular geometry of any given molecule. This structure helps in knowing the arrangement . Tingnan ang higit paThe electronic configuration of the Carbon atom in its ground state is 1s22s22p2, and that of an Oxygen atom is 1s22s2p4. When the electrons are in an excited state, they jump to other orbitals. In its excited state, the atom’s electronic configuration becomes . Tingnan ang higit paThe molecular Geometry of any compound is based on the arrangement of atoms, electron pairs, and bonds. Here in CO2, both Oxygen atoms form sigma bonds with the central . Tingnan ang higit pa
A quick explanation of the molecular geometry of CO2 including a description of the CO2 bond angles. We can see that there are only two atoms attached to the central Carbon (C) atom and.From the BP and LP interactions we can predict both the relative positions of the atoms and the angles between the bonds, called the bond angles. Using this information, we can describe .Two double bonds connect the carbon and oxygen atoms in the CO2 Lewis structure. Each oxygen atom must bond twice, and each carbon atom must bond four times, according to the octet rule.
Molecular Geometry Of CO2: How Lewis Structure Predicts Shape Of Molecules. The molecular geometry of a compound provides insight into its physical properties, chemical properties, and . The bond angle of CO2 is 180°. The molecular geometry of any compound can be determined by the VSEPR theory. The VSEPR chart is attached below, which will give us an idea about this. The Lewis structure of CO2 involves two oxygen atoms sharing double bonds with a central carbon atom. As we know that, C atom has 4 valence electrons and each O atom has 6 valence electrons. The total number of .
CO2 Bond Angle. The molecular geometry of the CO2 is linear and arranged like O = C = O, which makes the bond angle of CO2 = 180 degrees. Moreover, the planer-shaped geometry, . Bond Angles. Bond angles also contribute to the shape of a molecule. Bond angles are the angles between adjacent lines representing bonds. The bond angle can help . CO2 has a linear molecular geometry with a bond angle of 180° on a plan. Molar mass of CO2 is 44.01 g/mol which is also known as molecular weight. Carbon dioxide has an sp hybridization type because the steric .co2 bond angle and shape Sigma bonds are formed between Carbon and Fluorine. Pi-bonds are absent, making the structure remarkably stable. As such, the hybridization of the central Carbon atom is sp 3. CF4 Bond Angles. According to the VSEPR .
Carbon has sp3 hybridization, and the molecule takes up a tetrahedral shape to keep the repulsive forces of bonding pairs at a minimum. The bond angle of H-C-H is 109.5°. To know about the polarity of the CH4 .Bond angles also contribute to the shape of a molecule. Bond angles are the angles between adjacent lines representing bonds. The bond angle can help differentiate between linear, trigonal planar, tetraheral, trigonal-bipyramidal, and octahedral. The ideal bond angles are the angles that demonstrate the maximum angle where it would minimize . In its most stable state, the two Carbon atoms act as the central atoms in the structure. They form double bonds with each other and covalent bonds with the Hydrogen atoms. The hybridization of the Carbon atoms in C 2 H 4 is given by sp 2. C 2 H 4 has a Trigonal Planar molecular structure with bond angles of 121.3 °.Since the phosphorus is forming five bonds, there can't be any lone pairs. The 5 electron pairs take up a shape described as a trigonal bipyramid - three of the fluorines are in a plane at 120° to each other; the other two are at right angles to this plane. The trigonal bipyramid therefore has two different bond angles - 120° and 90°.CO 2 (Carbon dioxide) Lewis Structure and Shape. Carbon dioxide (CO 2) lewis structure has two oxygen atoms and one carbon atom. There are two double bonds around carbon atom in the CO 2.No lone pairs on carbon atom and each oxygen atom has . That’s the sp³ bond angle. The name for this 3-dimensional shape is a tetrahedron (noun), which tells us that a molecule like methane (CH4), or rather that central carbon within methane, is tetrahedral in shape. . each, set aside for the triple bond (2 pi bonds on top of the sigma). This makes HCN a Linear molecule with a 180° bond angle . From this we can easily draw the Lewis dot diagram of CO2 by adjusting two double bonds between carbon and oxygen (O=C=O). The molecular geometry of CO2 is linear with a bond angle of 180 ° because the dipole charges are canceled by each other as molecule is symmetrically arranged. Although both C=O bonds are polar but the entire molecule is .Bond angles also contribute to the shape of a molecule. Bond angles are the angles between adjacent lines representing bonds. The bond angle can help differentiate between linear, trigonal planar, tetraheral, trigonal-bipyramidal, and octahedral. The ideal bond angles are the angles that demonstrate the maximum angle where it would minimize .
This geometric shape is mainly due to the presence of a sigma bond and valence electron pairs repelling each other where they are forced to move to the opposite side of the carbon atom. As a result, the carbon atom acquires such a linear molecular shape with symmetric charge distribution. The carbon dioxide bond angle is 180 degrees.
According to the VSEPR model, the H - C - H bond angle in methane should be 109.5°. This angle has been measured experimentally and found to be 109.5°. Thus, the bond angle predicted by the VSEPR model is identical to that observed. We say that methane is a tetrahedral molecule. The carbon atom is at the center of a tetrahedron. A bond angle is the angle between any two bonds that include a common atom, usually measured in degrees. A bond distance (or bond length) is the distance between the nuclei of two bonded atoms along the straight line .

However, the Lewis structure provides no information about the shape of the molecule, which is defined by the bond angles and the bond lengths. For carbon tetrachloride, each C-Cl bond length is 1.78Å and each Cl-C-Cl bond .
Thus far, we have used two-dimensional Lewis structures to represent molecules. However, molecular structure is actually three-dimensional, and it is important to be able to describe molecular bonds in terms of their distances, angles, and relative arrangements in space (Figure 7.14).A bond angle is the angle between any two bonds that include a common atom, .
How Does Changing a Bond to a Double or Triple Bond Affect the Shape of the Molecules? In VSEPR theory, the more bonds there are around an atom's central core electrons in a chemical bond, the more distortion in bond angles. In essence, multiple bonds simply take up more space, just like with lone pairs of electrons.co2 bond angle and shape CO2 Lewis Structure, H2O Shape. The molecular shape of the H 2 O molecule is bent.. Concluding Remarks . To summarize this article we can say that the H 2 O molecule comprises two hydrogen atoms and one oxygen atom.; There are a total of 8 valence electrons for this molecule, out of which four are used to form O-H sigma bonds. Because it is forming 4 bonds, these must all be bonding pairs. Four electron pairs arrange themselves in space in what is called a tetrahedral arrangement. A tetrahedron is a regular triangularly-based pyramid. The carbon atom would be at the centre and the hydrogens at the four corners. All the bond angles are 109.5°.When determining the shape and bond angles of a molecule, the following VSEPR rules should be considered: . 6.7.2 Substituted Aromatic Carbon-carbon Bond Formation; 6.8 Organic Synthesis. 6.8.1 Techniques; 6.8.2 Synthetic Routes; 6.9 Analytical Techniques. 6.9.1 Thin Layer Chromatography, TLC;
The ground-state electronic configuration of iodine is [Kr] 4d 10 5s 2 5p 5. Upon excitation, iodine gains an extra electron and the configuration of the iodide (I –) ion becomes [Kr] 4d 10 5s 2 5p 6. During chemical bonding in ICl 4 –, two of the 5p electrons of iodine shift to its empty 5d atomic orbitals.
co2 bond angle and shape|CO2 Lewis Structure,